April 18th, 2016 was a life-changing day for people living with keratoconus in the USA; corneal collagen crosslinking, the revolutionary treatment that’s been performed in Europe since 1999, was finally approved by the FDA.
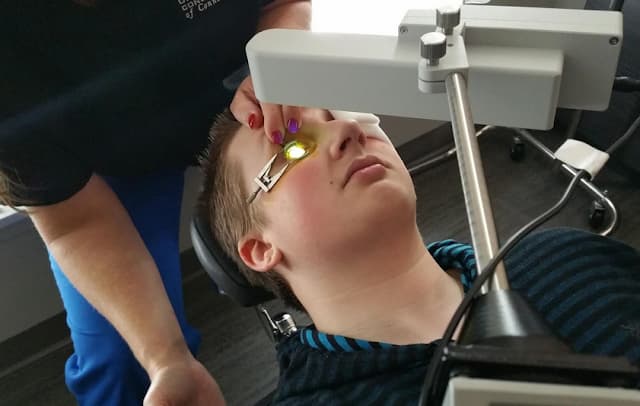
Keratoconus affects as many as 1 in every 500 people, causing potentially severe vision loss. But it’s progression and causes are poorly understood. During the disease process, the cornea tissue thins and weakens, causing the cornea to prolapse forward like a steepening mountain. The resulting warpage and associated scarring wrecks havoc on vision, often requiring severely invasive procedures like full thickness corneal transplant as the condition worsens. In corneal collagen crosslinking, the goal is to stop the progression of keratoconus while the damage is still mild and vision is still usable, long before more aggressive surgical interventions would be needed. Using riboflavin and UV light, doctors are able to strengthen the connections of the collagen fibers of the cornea so that they hold tighter and stronger together, locking the cornea in place.
Because the procedure is so new here in the USA, most people living with keratoconus are being offered corneal crosslinking as a surgical option for the first time. Confusion about the procedure and its possible outcomes abounds. I interviewed Dr. Christine B. Wisecarver, an optometrist practicing at See Clearly Vision who manages the care of patients before and after corneal crosslinking surgery, and their surgical coordinator, Irina Price about what patients need to know before corneal crosslinking.
Who do you consider a good candidate for corneal collagen crosslinking?
There has been a paradigm shift in the management of keratoconus patients. Patients with evidence of keratoconus should now be treated with collagen crosslinking as soon as possible. Once they have been cross linked, patients should be managed with spectacles and contact lenses as needed. Collagen crosslinking is currently FDA approved for the treatment of progressive keratoconus only. It is not currently covered by any insurance company, and much like refractive surgery, is considered at the discretion of the doctor. Candidates for crosslinking are patients who have been diagnosed with keratoconus and are cleared for crosslinking by their physician.
At what age would you consider treating a young patient with keratoconus that was advancing rapidly?
Safety and efficacy has not been established in patients under the age of 14 years old.
What can a patient expect visually from the procedure?
Crosslinking helps prevent progression of the disease. It does not reverse keratoconus, and is not a refractive procedure (meaning it will not correct a patient’s vision). Some patients get a mild flattening effect as they heal, but this does not happen for every patient. All crosslinking patients are advised that if they are wearing specialty contact lenses before the procedure, they will likely need them following their treatment. Since the cornea will be changing in the first 6 months after the procedure, a new refraction and contact lens refit will be needed.
Corneal collagen crosslinking does not improve vision in keratoconus patients. The sole purpose of crosslinking is to halt the progressive steepening and thinning of the cornea. Having said that, it is currently the ONLY treatment that can reduce and ultimately halt the progression of the disease.
What can a patient expect of the procedure itself?
The procedure is about 1 hour long with drops instilled every 2 minutes and UV light exposure to the cornea for 30 minutes. A bandage contact lens is then placed on the eye to allow the surface cells to heal. After 5 to 7 days, the bandage contact lens is removed. The healing time is very similar to PRK (except here patients get drops and UV light instead of laser). Patients may be uncomfortable for the first 3 days while the surface is healing. Antibiotic and steroid eye drops are used in the immediate post-operative period. Using a copious amount of preservative-free artificial tears can help to speed up the healing process.
How long after the procedure does it take to see results?
Patients will likely not appreciate any visual result from treatment. The cornea usually stabilizes (i.e., curvature changes and prescription changes) by the 6 month mark. After that time, topography can be used to demonstrate that the procedure has helped to slow the progression of keratoconus.
Besides keratoconus, are there other conditions you would treat with corneal crosslinking? There is no current FDA approval for treatment with corneal crosslinking beyond that of progressive keratoconus. Off-label treatments for corneal disorders other than keratoconus (for example, corneal ectasia following refractive surgery), are at the discretion of the corneal specialist.
What do you see for the future using corneal crosslinking technology?
Future indications for collagen crosslinking are currently being studied in the US and abroad. One possible indication is LASIK/PRK Xtra (crosslinking at the time of refractive surgery) to prevent post-refractive ectasia in higher risk patients like high myopes, young patients, and those with thin corneas. Additionally, surgeons are exploring targeted crosslinking where the aim is to achieve a visually improving refractive result in addition to preventing progression of keratoconus. Combination treatments like these, and the use of crosslinking for patients at risk for corneal perforation are areas in which cross linking may play a role in the future.




















